Introduction
Historically, nearly all relapses in classical Hodgkin lymphoma (cHL) occur within the first 5 years (Radford et al, BMJ 1997). In the phase 3 ECHELON-1 study (NCT01712490), treatment with brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) significantly improved modified progression-free survival (PFS) in patients (pts) with newly-diagnosed Stage III/IV cHL compared with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) (Connors et al, NEJM 2018). 3- and 4-year follow-up (Straus et al, Blood 2020; Bartlett et al, Blood 2019) reported durable PFS benefit with A+AVD vs ABVD in the intent-to-treat (ITT) population that was consistent across most key pt subgroups, irrespective of interim positron emission tomography (PET) scan status, disease stage, and baseline disease risk factor score. We report updated efficacy and safety results for pts in the ECHELON-1 study after a median follow-up of 55.6 months.
Methods
In this evaluation of longer follow-up, an exploratory analysis of PFS (time from randomization to relapse, progression, or death from any cause) per investigator (INV) was conducted, with a cutoff date of May 18, 2020. Pts with previously untreated Stage III or IV cHL were randomized 1:1 to receive up to six cycles of A+AVD (n=664) or ABVD (n=670) intravenously on days 1 and 15 of a 28-day cycle. An interim PET scan after cycle 2 (PET2) was required. Resolution and improvement (defined as improvement by ≥1 grade from worst grade as of the latest assessment) of peripheral neuropathy (PN) in pts with ongoing symptoms at the end of treatment (EoT) were monitored during the extended follow-up period. The rate of secondary malignancies, and the incidence and outcomes of pregnancies among pts and their partners were also assessed.
Results
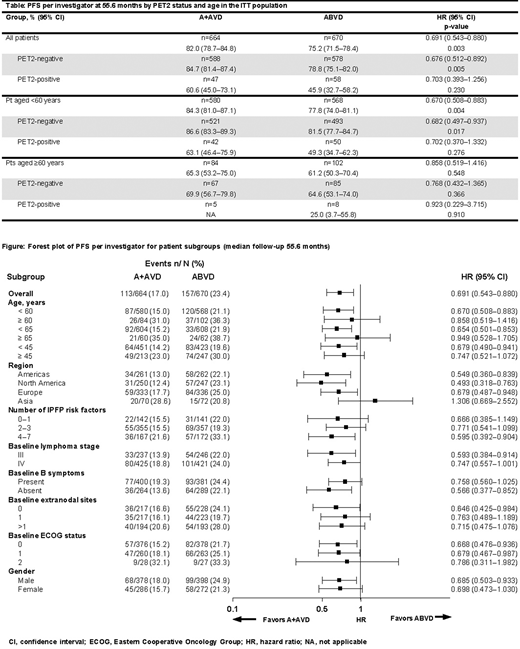
With extended follow-up (median 55.6 months; 95% CI 55.2-56.7) estimated 5-year PFS rates were 82.0% (95% CI 78.7-84.8) for A+AVD and 75.2% (95% CI 71.5-78.4) for ABVD. Overall, PFS per INV favored A+AVD over ABVD (HR 0.691; 95% CI 0.543-0.880; p=0.003) (Table). Exploratory subgroup analyses by PET2 status and age demonstrated PFS benefits regardless of PET2 status (Table); estimated 5-year PFS per INV with A+AVD vs ABVD in the ITT population was 84.7% vs 78.8% in PET2-negative pts (HR 0.676; 95% CI 0.512-0.892; p=0.005), and 60.6% vs 45.9% in PET2-positive pts (HR 0.703; 95% CI 0.393-1.256; p=0.230). PFS benefit with A+AVD over ABVD was also independent of the number of International Prognostic Factors Project (IPFP) risk factors (Figure). After a median follow-up of almost 5-years, 84% (370/442) and 86% (245/286) of pts with treatment-emergent PN reported complete resolution or improvement of symptoms in the A+AVD and ABVD arms, respectively. Median time to complete resolution of PN events that were ongoing at EoT was 30 weeks (range 0-262) in the A+AVD arm and 16 weeks (range 0-267) in the ABVD arm; median time to improvement was 49 weeks (range 8-270) and 12 weeks (range 2-70) , respectively. Of the 132 (30%) pts with ongoing PN in the A+AVD arm, 77 (17%), 39 (9%), 15 (3%) and 1 (<1%) experienced a maximum severity of grade 1, 2, 3 or 4, respectively. In the ABVD arm, PN was ongoing in 61 pts (21%); maximum severity was grade 1, 2, 3 or 4 in 40 (14%), 17 (6%), 4 (1%) and 0 pts, respectively. A total of 124 pregnancies were reported among pts and their partners (40 female and 30 male pts in the A+AVD arm; 26 female and 28 male pts in the ABVD arm). The proportion of live births was similar between arms for female pts (26/40 in the A+AVD arm and 17/26 in the ABVD arm, 65% in each arm) and for male pts' partners (19/30 [63%] in the A+AVD arm and 20/28 [71%] in the ABVD arm). No stillbirths were recorded. Additional follow-up at an estimated median of ~5 years and secondary malignancy data will be presented.
Conclusions
After a median follow-up of 55.6 months, A+AVD continues to demonstrate a robust and durable treatment benefit independent of disease stage, risk factor score, and PET2 status. In addition, compared with ABVD, treatment adaptation by interim PET2 status is not required and bleomycin exposure is avoided. The sustained PFS benefit with A+AVD is coupled with a manageable safety profile with symptoms of PN improving or resolving over time and similar pregnancy rates in both treatment arms. The benefits observed with A+AVD at this important milestone suggest that A+AVD is an attractive treatment option for all pts with previously untreated Stage III or IV cHL.
Straus:Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Imedex, Inc.: Speakers Bureau; Targeted Oncology: Consultancy, Speakers Bureau; NY Lymphoma Rounds: Consultancy; Takeda Pharmaceuticals: Research Funding, Speakers Bureau; OncLive: Speakers Bureau; Elsevier: Membership on an entity's Board of Directors or advisory committees, Other: CME writer; ASH: Other: Conference in December 2019 on HL to other physicians during ASH; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Connors:Seattle Genetics: Other: Sponsorship to educational presentations; Takeda: Other: Sponsorship to educational presentations. Illés:Takeda, Seattle Genetics: Research Funding; Novartis, Janssen, Pfizer, Roche;: Other: Travel, Accommodations, Expenses; Janssen, Celgene, Takeda, Novartis Pharma SAS, Pfizer Pharmaceuticals Israel, Roche;: Consultancy, Honoraria; Celgene, Janssen, Novartis,Roche, Takeda: Consultancy. Lech-Marańda:Roche, Novartis, Takeda, Janssen-Cilag, Amgen, Gilead, AbbVie, Sanofi: Consultancy; Roche, Amgen, Gilead: Speakers Bureau. Feldman:KITE: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Morphosis: Other: Ad board; AstraZeneca: Other: Ad board; BMS/Celgene: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Consultancy, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Pharmacyclics: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau. Smolewski:Roche Poland: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Sandoz: Honoraria; Morphosis: Honoraria. Savage:Roche (institutional): Research Funding; Merck, BMS, Seattle Genetics, Gilead, AstraZeneca, AbbVie, Servier: Consultancy; BeiGene: Other: Steering Committee; Merck, BMS, Seattle Genetics, Gilead, AstraZeneca, AbbVie: Honoraria. Bartlett:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Consultancy, Research Funding; Roche/Genentech: Consultancy, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; BTG: Consultancy; Acerta: Consultancy; Affimed Therapeutics: Research Funding; ADC Therapeutics: Consultancy; Autolus: Research Funding; BMS/Celgene: Research Funding; Forty Seven: Research Funding; Immune Design: Research Funding; Janssen: Research Funding; Kite, a Gilead Company: Research Funding; Merck: Research Funding; Millennium: Research Funding. Walewski:Gilead: Consultancy, Honoraria; Servier: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Other: Travel Support, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; GSK: Research Funding. Ramchandren:Janssen: Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding. Zinzani:Eusapharma: Consultancy, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Immune Design: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Consultancy, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Immune Design: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics, Inc.: Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; EUSA Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kirin Kyowa: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Hutchings:Celgene, Genmab, Janssen, Novartis, F. Hoffmann-La Roche, Takeda: Research Funding; Genmab, F. Hoffmann-La Roche, Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Munoz:Juno/Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Alexion: Consultancy; Beigene: Consultancy, Speakers Bureau; Fosunkite: Consultancy; Innovent: Consultancy; Acrotech/Aurobindo: Speakers Bureau; Verastem: Speakers Bureau; AstraZeneca: Speakers Bureau; Kyowa: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Genentech/Roche: Research Funding, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau; Merck: Research Funding; Portola: Research Funding; Incyte: Research Funding; Millenium: Research Funding; Pfizer: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Bayer: Consultancy, Research Funding, Speakers Bureau. Kim:JJ: Research Funding; Celltrion: Research Funding; Kyowa Kirn: Research Funding; Donga: Research Funding; Mundipharma: Research Funding; Pfizer: Research Funding; F. Hoffmann-La Roche: Research Funding. Advani:Astra Zeneca, Bayer Healthcare Pharmaceuticals, Cell Medica, Celgene, Genentech/Roche, Gilead, KitePharma, Kyowa, Portola Pharmaceuticals, Sanofi, Seattle Genetics, Takeda: Consultancy; Celgene, Forty Seven, Inc., Genentech/Roche, Janssen Pharmaceutical, Kura, Merck, Millenium, Pharmacyclics, Regeneron, Seattle Genetics: Research Funding. Ansell:Bristol Myers Squibb: Research Funding; Takeda: Research Funding; ADC Therapeutics: Research Funding; AI Therapeutics: Research Funding; Trillium: Research Funding; Affimed: Research Funding; Regeneron: Research Funding; Seattle Genetics: Research Funding. Younes:HCM: Consultancy; Daiichi Sankyo: Consultancy; Curis: Consultancy; BMS: Consultancy; Novartis: Consultancy; Epizyme: Consultancy; BioPath: Consultancy; AstraZeneca: Current Employment; Janssen: Consultancy; Takeda: Consultancy. Gallamini:Takeda: Other: Speaker. Liu:Takeda Pharmaceuticals: Current Employment. Little:Takeda: Current Employment, Current equity holder in publicly-traded company. Fenton:Seattle Genetics: Current Employment, Current equity holder in publicly-traded company. Fanale:Seattle Genetics: Current Employment, Current equity holder in publicly-traded company. Radford:GlaxoSmithKline: Current equity holder in publicly-traded company, Other: Spouse; AstraZeneca: Current equity holder in publicly-traded company, Other: Spouse; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Research Funding; ADCT: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal